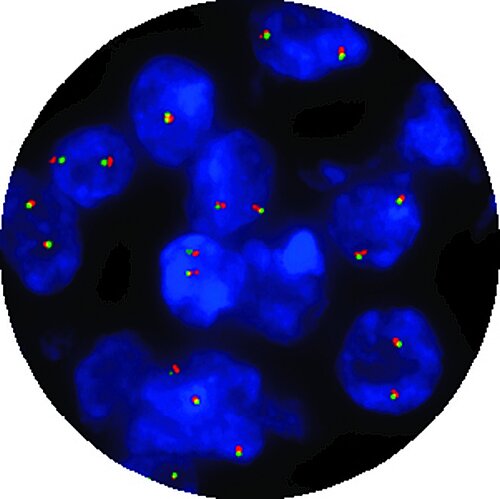
CytoCell® IVDR probes
IVDR-certified FISH probes
- Available now! The first IVDR-certified probes on the market
- Safe, reliable and effective products for you and your patients
- Haematological cancers and pre-natal conditions
In 1991, CytoCell® became the first provider of FISH probes in the world. Over 30 years later, they are still pioneering the FISH frontiers and are the first to market with In Vitro Diagnostic Regulation (IVDR) certified FISH Probes!
Switching to IVDR-certified FISH probes means you can be confident that your laboratory is using safe, reliable and effective tools for diagnosing patients.
CytoCell® IVDR FISH probes remain the same trusted products with the extra IVDR seal — the unquestionable testament to our commitment to provide innovative, class-leading products under this substantially more stringent regulation.
Thirteen CytoCell® FISH probes that are important for the patient management of haematological cancers and pre-natal conditions have been IVDR-certified and are available to order. OGT will continue to pursue additional certifications for our CytoCell® FISH probe portfolio.
| Haematology probes | Product code |
| AML1 (RUNX1) Breakapart Probe | CE-LPH027 |
| AML1/ETO (RUNX1/RUNX1T1) Translocation, Dual Fusion Probe | CE-LPH026 |
| BCR/ABL (ABL1) Translocation, Dual Fusion Probe | CE-LPH007 |
| BCR/ABL (ABL1) Plus Translocation, Dual Fusion Probe | CE-LPH038 |
| CBFB Breakapart Probe | CE-LPH 089 |
| CKS1B/CDKN2C (P18) Amplification/Deletion Probe | CE-LPH039 |
| Del(5q) Deletion Probe | CE-LPH024 |
| Del(7q) Deletion Probe | CE-LPH025 |
| Del(20q) Deletion Probe | CE-LPH020 |
| FAST PML/RARα (RARA) Translocation, Dual Fusion Probe | CE-LPH064 |
| IGH/MAF Plus v2 Translocation, Dual Fusion Probe | CE-LPH108 |
| MLL (KMT2A) Breakapart Probe | CE-LPH013 |
| Prenatal probes | |
| Prenatal 13 and 21 Enumeration Probe Kit | CE-LPA003 |
Switch to a CytoCell® IVDR-certified FISH probe now and be assured that you won’t need to consider it again. Should you need any support, our expert team is always on hand to guide you through the transition.
Start the process early – contact your local Sysmex representative and request an evaluation kit today!
View all CytoCell ancillary products
CytoCell Tissue Pretreatment Kit
OGT’s CytoCell® IVDR-certified range of fluorescence in situ hybridisation (FISH) probe kits are in vitro diagnostic (IVD) medical devices for the detection of prenatal trisomy 13 & 21 and acquired cancer-related chromosome alterations.
They have been CE-marked under Regulation (EU) 2017/746 (IVDR) as Class C IVD medical devices for laboratory professional use only and are not intended for use as a standalone diagnostic or companion diagnostic.
Refer to each individual FISH probe kit’s Instructions for use for their specific Intended purpose, indications, and limitations.”
| Probe | 50µl per vial (5 tests) or 100µl per vial (10 tests), provided in hybridisation solution and are ready to use. |
| Counterstain | 150µl per vial (15 tests), DAPI antifade |
| Storage temperature | -25°C to -15°C, in the dark |
Sysmex Nordic ApS Suomen sivuliike
KPY Novapolis
Microkatu 1
70210 Kuopio
Finland
+358 (0) 102999940